 An anti-adhesive technology that prevents the formation of blood clots and bio-films on a wide range of different medical surfaces using coating materials already approved for medical applications Every device implanted in the body or in contact with flowing blood faces two critical challenges that can threaten the life of the patient it is meant to help: blood clotting and bacterial infection. Thin Layer Perfluorocarbon (TLP) coating specifically designed to prevent clot formation and bio-film formation when adhered to existing medical devices. The coating consists of a chemically inert Perfluorocarbon material that is already approved by the Food and Drug Administration (FDA) for applications such as liquid ventilation, blood substitution, eye surgery, and more.
An anti-adhesive technology that prevents the formation of blood clots and bio-films on a wide range of different medical surfaces using coating materials already approved for medical applications Every device implanted in the body or in contact with flowing blood faces two critical challenges that can threaten the life of the patient it is meant to help: blood clotting and bacterial infection. Thin Layer Perfluorocarbon (TLP) coating specifically designed to prevent clot formation and bio-film formation when adhered to existing medical devices. The coating consists of a chemically inert Perfluorocarbon material that is already approved by the Food and Drug Administration (FDA) for applications such as liquid ventilation, blood substitution, eye surgery, and more.
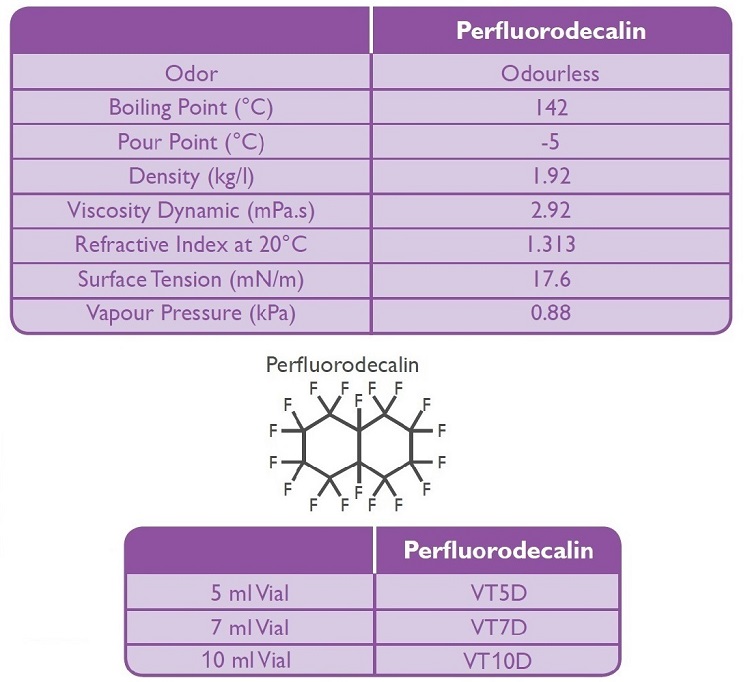
• Contains more than 98% Perfluorodecaline or Perfluoro-octane
• Purified Perfluorodecaline or Perfluoro-octane sterile and apyrogenic.
• Transparent, colourless and optically clear
• Both products have clear differentiation with high specific gravity
• Presented in 5-7-10 ml glass vials syringe depending on the surgeons preferences
• Prepared in a full automated system
• Has a colour code on the packet side to be able to store at room temperature and recognize easily